Latest study on Mpox vaccination highlights the need for booster doses
Published: 2026-01-08
Over the past several years, the global community has faced repeated reminders of the threat posed by emerging and re-emerging infectious diseases. Mpox (formerly monkeypox) is a zoonotic disease caused by an orthopoxvirus related to variola virus (the causative agent of smallpox). It can be classified into two clades (clades 1 and 2), that are further subdivided into two subclades (1a and 1b, and 2a and 2b). See our mpox topic for more general information on the virus. It gained global attention during the 2022–2023 outbreak, where cases were detected in Europe and North America.
Vaccination strategies were rapidly implemented in response to the outbreak. Sweden provided a dose-sparing intradermal-only regimen of the Modified Vaccinia Ankara–Bavarian Nordic (MVA-BN) vaccine to at-risk populations (primarily gay men, bisexual men, and other men that have sex with men (GBMSM)). The vaccination is provided either as two intradermal doses (0.1 mL) at least 28 days apart, or a single dose for individuals with prior smallpox vaccination. Intradermal administration and dose-sparing vaccination strategies were widely adopted in Sweden after 1976, when the smallpox vaccination was removed from childhood vaccination regimens. However, questions remain regarding the immunology induced by these regimens and whether booster vaccinations are required.
Espinosa-Gongora et al. (2025) conducted a longitudinal study to assess mpox-specific neutralising antibodies up to 9 months following 1 or 2 doses of intradermal MVA-BN vaccination in Sweden. The study focused on individuals at increased risk for mpox infection, and aimed to find insights into vaccine-induced immunity over time.
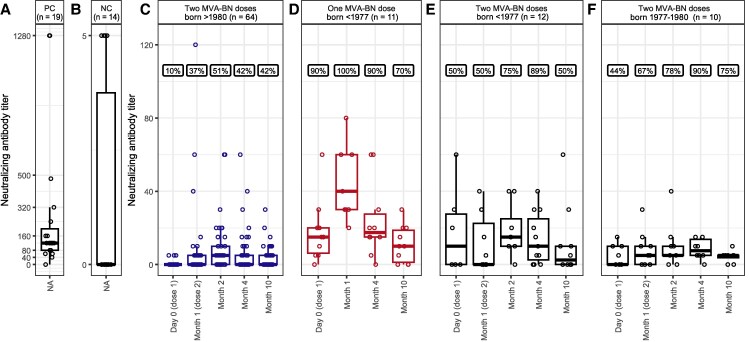
The researchers followed a prospective longitudinal cohort of participants (from the at-risk population, GBMSM) receiving either one or two intradermal doses of the MVA-BN vaccine. Serum samples were collected at 5 different time points, from before vaccination and up to 9 months after the final dose, allowing for a detailed assessment of antibody kinetics. Neutralising antibodies against Mpox clade 2b (which was responsible for the outbreak in 2022-23), were measured using live-virus micro neutralisation assays performed in Vero cell culture, a gold-standard approach for evaluating functional antibody responses. Statistical analyses, including paired comparisons and generalised linear mixed models, were used to evaluate changes over time and identify factors influencing antibody levels.
The results showed that neutralising antibody responses peaked approximately 1 month after vaccination, followed by a significant decline between 3 and 9 months. Importantly, individuals with evidence of prior smallpox vaccination (inferred from age and vaccination history) exhibited higher antibody responses. This highlights the role of immunological memory in shaping vaccine outcomes. Although antibody levels at later time points often remained above baseline, the observed waning raises questions about the duration of protection conferred by intradermal MVA-BN vaccination alone.
Together, these findings contribute valuable real-world data on vaccine efficacy, immune memory, and antibody waning following mpox vaccination. By combining longitudinal sampling with robust neutralisation assays, the study provides an evidence base that can inform booster strategies, vaccination policies, and future outbreak preparedness efforts. As global health agencies continue to refine responses to emerging viral threats, such data are critical for optimising vaccination approaches and sustaining population-level protection.
Data
- Demographic and clinical characteristics of the study population along with the results of comparing analysing the role of each MVA-BN vaccination dose based on paired Wilcoxon rank test are available in the supplementary materials.
Article
DOI: 10.1093/ofid/ofaf657
Espinosa-Gongora, C., Christ, W., Mayola Danés, N., Eichler-Jonsson, C., Filén, F., Storgärd, E., Westergren, V., Klingström, J., Gredmark-Russ, S., Johansen, K., Ekström, AM., & Sondén, K. (2025, November). Mpox-Specific Neutralizing Antibodies up to 9 Months Following 1 or 2 Doses of Intradermal MVA-BN Vaccination in Sweden. Open Forum Infectious Diseases (Vol. 12, No. 11, p. ofaf657). US: Oxford University Press.
Funding
Swedish Medical Society (grant number 999710).