Host range expansion in Giardia duodenalis linked to Muller's Ratchet
Published: 2026-01-29
Giardia duodenalis is an intestinal protozoan parasite that infects a wide variety of vertebrate hosts, including humans, where it causes the diarrheal disease giardiasis. Transmission occurs via ingestion of hardy cysts in contaminated water, food, or through direct contact. Despite its simple cellular organisation, G. duodenalis exhibits remarkable genetic diversity that underpins its capacity to infect different hosts. The species is divided into several genetically distinct assemblages, many of which display strict host specificity, making it an excellent system for studying how parasites adapt and switch hosts.
Classical evolutionary theory predicts that parasites that reproduce strictly asexually will struggle to adapt to changing environments, and will accumulate harmful mutations over time. This process is known as Muller’s Ratchet. However, G. duodenalis (and some other strictly asexually reproducing parasites) defy these expectations, successfully colonising new hosts. Tichkule et al. (2025) studied G. duodenalis to find a genomic explanation for how this can occur.
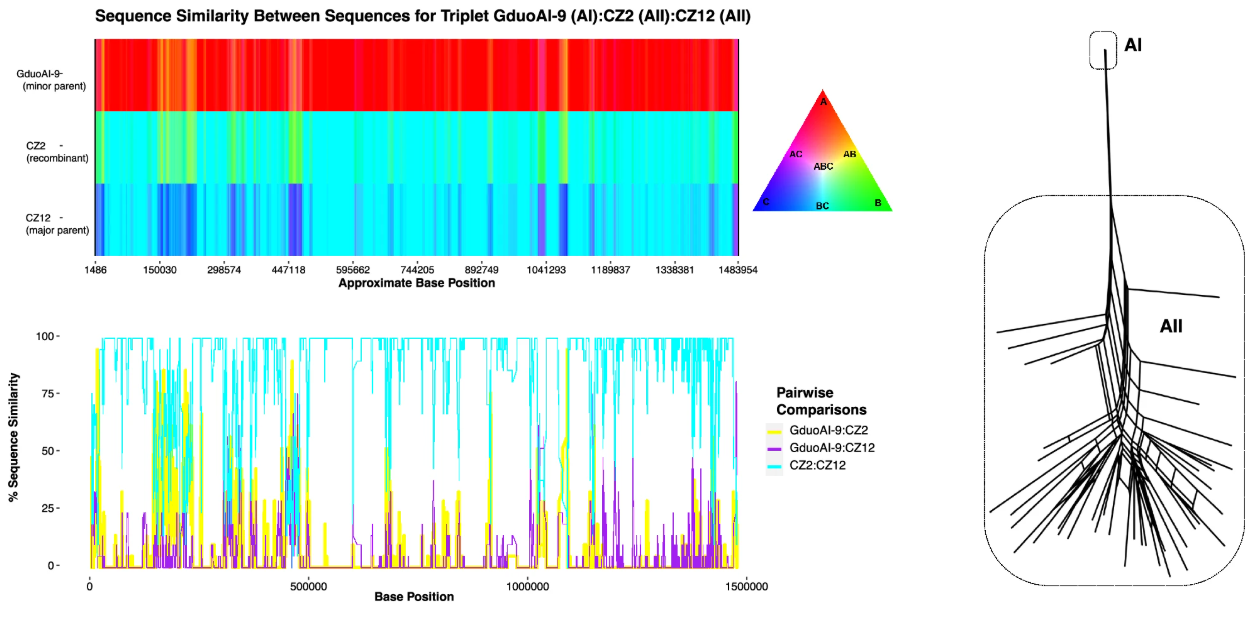
Tichkule et al. (2025) analysed whole-genome sequences from 107 G. duodenalis isolates belonging to Assemblage A, after excluding mixed infections. The isolates used were obtained from a wide range of hosts, including humans, dogs, cats, pigs, beavers, and sheep, and the environment. Comparative genomic analyses revealed two major subassemblages: AI and AII. Subassemblage AII showed evidence of meiotic recombination and was almost exclusively associated with humans. In contrast, subassemblage AI appeared to represent a more recently derived asexual lineage, lacking signs of recombination, but capable of infecting a broader spectrum of host species.
To clarify the evolutionary structure within Assemblage A, Tichkule et al. (2025) applied phylogenetic clustering and genomic distance metrics.This led to the identification of three genetic groups, including one intermediate cluster derived from feline and environmental isolates. This population framework enabled a fine-scale comparison of recombination patterns and mutation accumulation between the subassemblages. Subassemblage AI genomes displayed signatures typical of asexual evolution, such as strong linkage disequilibrium and a uniform mutation distribution across the genome. On the contrary, subassemblage AII showed evidence of recombination and decay of linkage consistent with sexual reproduction.
The asexual AI genomes carried an accumulation of deleterious mutations without the typical signs of adaptive selection. This mutational load is characteristic of Muller’s Ratchet, and may have reduced some host-specific molecular adaptations. As a result, AI parasites may have temporarily expanded their host range by losing specialisation constraints that normally restrict infection to specific hosts. However, Tichkule et al. (2025) suggest that this phase of broadened host range might be a transient stage before fitness decreases under mutational pressure.
By integrating evolutionary theory with population genomics, Tichkule et al. (2025) provides a mechanistic link between mutation accumulation and ecological flexibility in parasitic protozoa. It challenges the conventional view that asexuality necessarily limits pathogen adaptability, and demonstrates how genomic decay processes can paradoxically facilitate short-term expansion across host boundaries. These findings have broad implications for understanding host specificity, parasite persistence, and the evolutionary dynamics of emerging asexual pathogens.
Data
- Sequencing data is available at NCBI Sequence Read Archive (SRA) under the BioProject PRJNA1110996.
- Phylogenetic data in Newick format is available.
Article
DOI: 10.1038/s41467-025-65843-4
Tichkule, S., van Oosterhout, C., Cacciò, S. M., Weisz, F., Balan, B., Nohýnková, E.,Naung, M., Ansell, BRE., Emery-Corbin, SJ., Baker, L., Lalle, M., Svärd, S., Gasser, RB., & Jex, A. R. (2025). Host range expansion of asexual parasites can be explained by loss of adaptions in Muller’s Ratchet. Nature Communications, 16(1), 10805.